This paper reviews the sensitivity and specificity of RDTs for detecting P. falciparum and P. vivax in two different settings ¿ at health centres and in a household survey. The study includes a large number of patients (2,394 participants) and provides useful additional information about the performance of these RDTs.
Woyessa A, Deressa W, Ali A, Lindtjørn B. Evaluation of CareStartTM malaria Pf/Pv combo test for Plasmodium falciparum and Plasmodium vivax malaria diagnosis in Butajira area, south-central Ethiopia. Malaria Journal 2013, 12:218 doi:10.1186/1475-2875-12-218
Abstact:
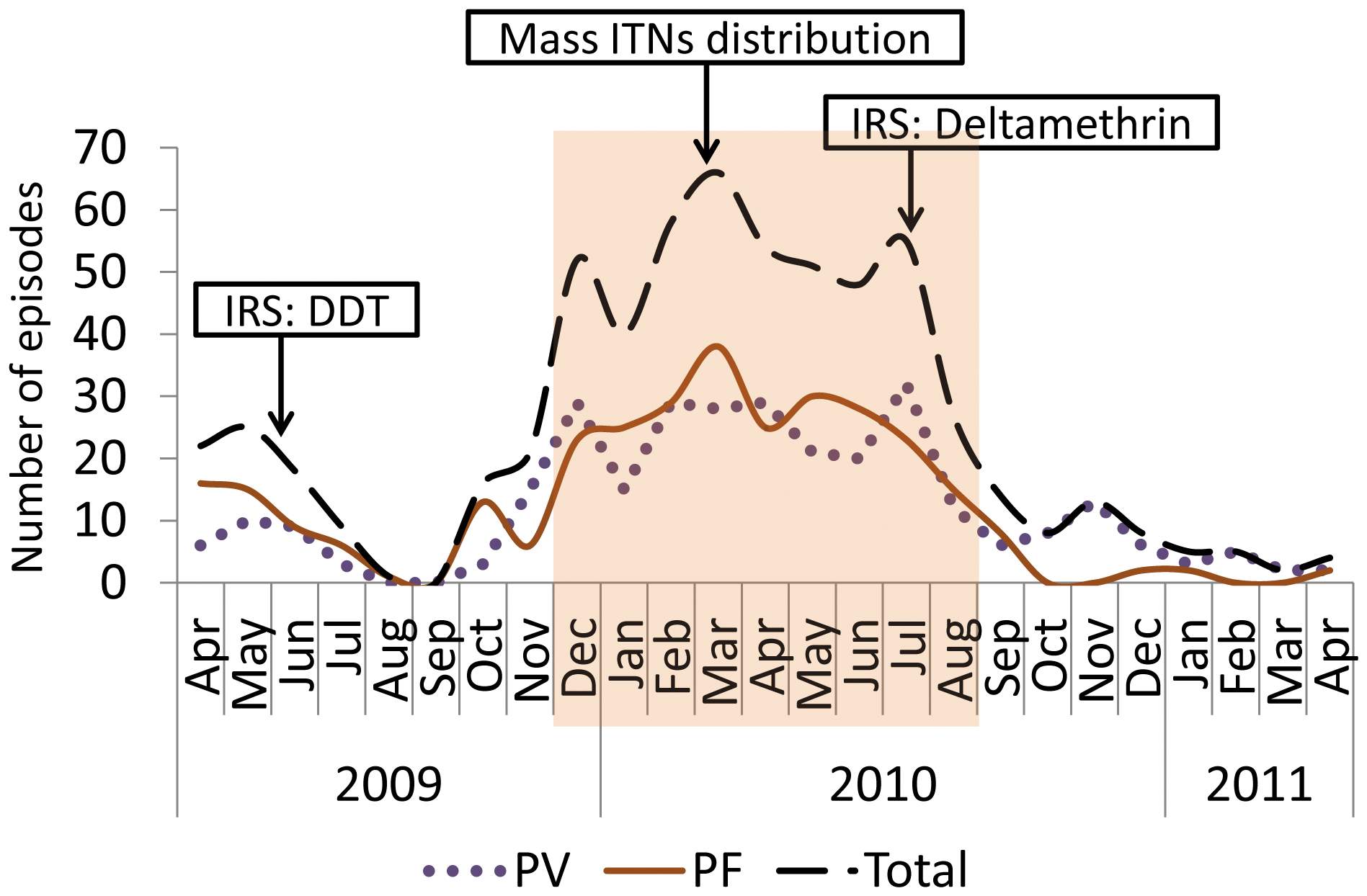
Malaria is a major public health problem in Ethiopia. Plasmodium falciparum and Plasmodium vivax co-exist and malaria rapid diagnostic test (RDTs) is vital in rendering parasite-confirmed treatment especially in areas where microscopy is not available. CareStartTM Malaria Pf/Pv combo test was evaluated compared to microscopy in Butajira area, south-central Ethiopia. This RDT detects histidine-rich protein-2 (HRP2) found in P. falciparum, and Plasmodium enzyme lactate dehydrogenase (pLDH) for diagnosis of P. vivax 2008-2010. The standard for the reporting of diagnostic accuracy studies was complied. Among 2,394 participants enrolled, 10.9% (n=87) were Plasmodium infected (household survey) and 24.5% (n=392) using microscopy. In the household surveys, the highest positivity was caused by P. vivax (83.9%, n=73), P. falciparum (15.0%, n=13), and the rest due to mixed infections of both (1.1%, n=1). In health facility, P. vivax caused 78.6% (n=308), P. falciparum caused 20.4% (n=80), and the rest caused by mixed infections 1.0% (n=4). RDT missed 9.1% (n=8) in household and 4.3% (n=17) in health facility-based surveys among Plasmodium positive confirmed by microscopy while 3.3% (n=24) in household and 17.2% (n=208) in health facility-based surveys were detected false positive. RDT showed agreement with microscopy in detecting 79 positives in household surveys (n=796) and 375 positives in health centre survey (n=1,598).RDT performance varied in both survey settings, lowest PPV (64.3%) for Plasmodium and P. falciparum (77.2%) in health centres; and Plasmodium (76.7%) and P. falciparum (87.5%) in household surveys. NPV was low in P. vivax in health centres (77.2%) and household (87.5%) surveys. Seasonally varying RDT precision of as low as 14.3% PPV (Dec. 2009), and 38.5% NPV (Nov. 2008) in health centre surveys; and 40-63.6% PPV was observed in household surveys. But the influence of age and parasite density on RDT performance was not ascertained. Establishing quality control of malaria RDT in the health system in areas with low endemic and P. falciparum and P. vivax co-exist is recommendable. CareStartTM RDT might be employed for epidemiological studies that require interpreting the results cautiously. Future RDT field evaluation against microscopy should be PCR corrected.

 Background
Background